Rebecca Johnson, PhD, Medical Analysis Recruitment and Inclusion Govt and Strategist
Key Takeaways
- Progress towards inclusive trials with out mandates. Even earlier than federal mandates similar to FDORA (2022), stakeholders throughout {industry}, academia, and advocacy started adopting practices to enhance illustration in medical trials—similar to journal insurance policies, group engagement, and sponsor-led variety objectives.
- New federal insurance policies could undermine beneficial properties. Latest federal actions—together with government orders limiting DEI initiatives and funding cuts—danger reversing progress by weakening infrastructure, discouraging innovation, and narrowing affected person entry to analysis alternatives.
- Sustainable change requires intentionality and accountability. Regardless of enhancements, gaps in illustration persist, particularly amongst rural, Indigenous, disabled, and LGBTQIA+ populations. Voluntary, stakeholder-led methods—backed by clear planning, cultural competency, and group engagement—are key to creating inclusion the norm throughout the medical analysis panorama.
The FDA has more and more issued pointers and set expectations for {industry} to enroll clinically related and numerous trial populations.1 Previous to the enactment of laws by the Meals and Drug Omnibus Reform Act of 2022, there have been no widespread mandates requiring consultant enrollment in industry-sponsored research. Actually, throughout a cross-sector medical analysis assembly in 2011, representatives acknowledged {industry} would take initiative and try to enhance the representativeness of medical analysis with out the necessity for regulatory mandates.2 It seems that many key gamers within the medical trial ecosystem did precisely that—implementing insurance policies and practices that straight or not directly help extra inclusive and consultant medical trials, similar to:
Journal insurance policies. As of January 2022, The New England Journal of Drugs started requiring authors to report on illness background and examine inhabitants representativeness—an method now adopted by many journals.3
FDA PFDD initiative. Launched in 2012, the FDA’s Affected person-Targeted Drug Growth (PFDD) program launched affected person listening periods and {industry} steering for incorporating affected person voice into medical product improvement.4,5 Though PFDD started in response to a congressional mandate below FDASIA, FDA remained dedicated to enhancing this initiative.6,7
Affected person advocacy efforts. Guided by the PFDD framework, advocacy teams have led over 100 externally hosted affected person listening periods.7
Sponsor-led inclusion objectives. Firms similar to Bristol Myers Squibb have set objectives to conduct trials at websites situated in racially and ethnically numerous metro areas to enhance accessibility.8
Federal coverage on trial participation protection. US coverage now requires Medicare, all state Medicaid packages, and personal insurers to cowl routine care prices for trial members.9,10
Cross-sector partnership launches EQBMED. Initially funded by PhRMA and established as a cross-sector collaborative, Equitable Breakthroughs in Drugs Growth (EQBMED) launched a pilot to develop an infrastructure of community-based trial websites.
Well being system DEI initiatives. Many well being methods have adopted variety, fairness, and inclusion (DEI) and well being fairness packages to higher mirror and accomplice with their communities.12,13
MRCT instruments for inclusion. The Multi-Regional Medical Trials (MRCT) Heart of Brigham and Ladies’s Hospital and Harvard has issued steering and toolkits to advertise illustration in analysis throughout dimensions similar to race, ethnicity, incapacity, LGBTQIA+ id, and extra.14
In the meantime, mandates for medical trial variety are starting to take impact. Except modified, the present laws enacted below FDORA will quickly require sponsors to submit variety motion plans (DAPs) to the FDA for all pivotal research.15 Encouragingly, many sponsors have already begun submitting DAPs voluntarily forward of those statutory necessities.16
DAPs supply a useful framework for proactively planning inclusive analysis throughout the event lifecycle and must be thought of greatest apply—no matter authorized obligation. They’ll information trial design, website choice and employees coaching, culturally tailor-made recruitment and retention methods, and ongoing engagement.
We seem like shifting in the best course towards sustainable change in medical trial inclusivity—even within the absence of obligatory insurance policies. In response to varied revealed studies, medical trials supporting FDA approvals have proven progress in demographic variety, with elevated illustration of ladies, older adults, and Black members, and a decline in White overrepresentation—dropping under 75% by 2020.17,18,19 Nevertheless, illustration stays inconsistent throughout research, with some populations over- or underrepresented relying on the situation and benchmark used.20,21
Regardless of ongoing variability in enrollment by demographic subgroup, progress is clear in comparison with 2011, when 83% of members in home industry-funded trials have been reportedly non-Hispanic White.2 Nevertheless, a lot of the main focus thus far has been on demographic traits. At a 2024 Nationwide Academies of Sciences, Engineering, and Drugs workshop, FDA and NIH leaders acknowledged progress in racial, ethnic, and sex-based inclusion, however emphasised that illustration stays missing throughout a broader spectrum, together with rural populations, older adults, Indigenous peoples, folks with disabilities, and sexual and gender-diverse people.16
In January 2025, new disruptions emerged with the administration’s government orders and effectivity measures—banning DEI initiatives, threatening investigations into “unlawful DEI practices” within the non-public sector, considerably slicing funding for analysis grants, and eliminating or downsizing a number of Well being and Human Providers divisions targeted on addressing well being disparities.22-26
Staffing and coverage modifications on the FDA, together with efforts to decrease prescription drug prices, have prompted some biotech corporations to maneuver early-stage trials abroad and will discourage funding in innovation and extra indications—resulting in fewer alternatives to supply medical analysis as a care choice.27,28,29 Collectively, these shifts threaten to erode progress in inclusive analysis—lowering investments in innovation, narrowing affected person entry, and weakening the infrastructure wanted to maintain equitable participation—and, extra broadly, pose dangers to medical improvement that rely on numerous participation for real-world relevance.
These should not the insurance policies the medical trial ecosystem had hoped for.
Whereas coverage ought to help reasonably than hinder progress, the query stays whether or not mandates are really efficient. Though the NIH has required the inclusion of ladies and members of numerous teams and their subpopulations—distinguished by racial, ethnic, or cultural heritage—in NIH-funded medical analysis because the 1993 Revitalization Act, many researchers fall brief in planning the right way to meet these objectives.30,31,32
Many NIH-funded principal investigators don’t set up recruitment objectives for all demographic subgroups, suggesting an absence of intentional planning. In a single examine, revealed in Annals of Epidemiology, fewer than 3% of Nationwide Coronary heart, Lung, and Blood Institute-funded researchers set objectives for all demographic teams and have been extra prone to miss objectives for Black (51%), Asian (55%), and Hispanic (44%) members than for White members (30%). In a associated examine, revealed in Journal of the Nationwide Most cancers Institute, solely 36% required cultural competency coaching for website employees, regardless of recommending it as a greatest apply.32
The implications are clear: disparities in trial participation persist in NIH-funded analysis. Participant demographics differ broadly throughout NIH institutes and facilities, with Hispanic people showing to be essentially the most underrepresented.17 Developments from Nationwide Most cancers Institute trials between 2005 and 2020 present decrease participation by older adults, ladies, Asian or Pacific Islander, American Indian or Alaska Native, and Hispanic people in contrast with their illustration within the US most cancers inhabitants.33
NIH initiatives, nonetheless, display how accountability can drive inclusive analysis practices. For instance, to keep up federal designation, Alzheimer’s illness analysis facilities should interact in ongoing group schooling—reaching numerous populations, together with non-English audio system.34 Group-building efforts similar to these from EQBMED and different sponsor—or site-led initiatives—are increasing however extra efforts are wanted. Sponsors and CROs might undertake a points-based system for website efforts and allocate assets to help site-level group engagement. NIH additionally requires institutional assessment boards to contemplate each its inclusion coverage and the FDA’s (now-withdrawn) steering on evaluating gender variations in drug analysis.30
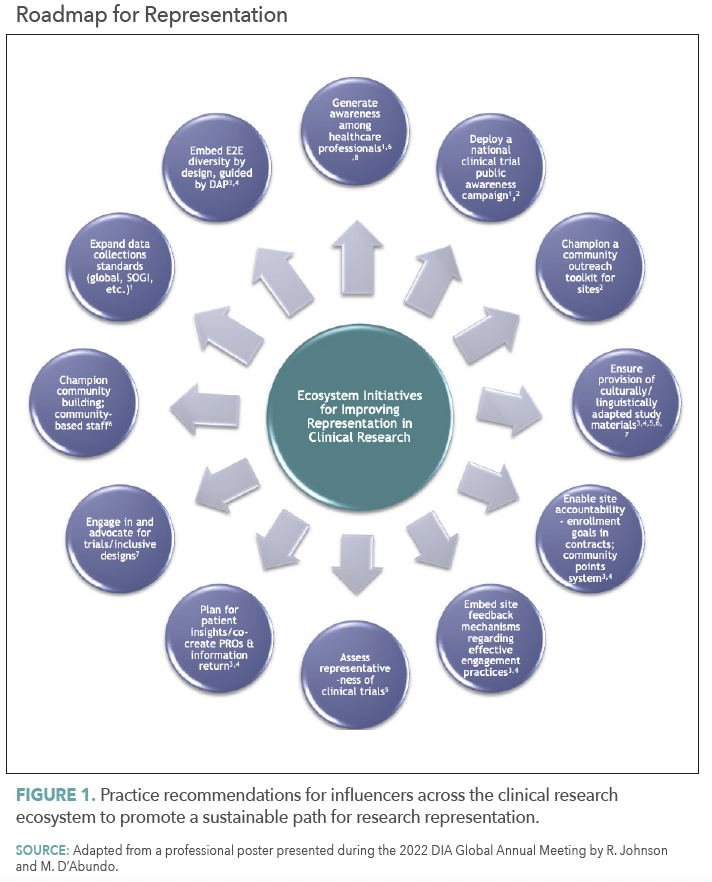
Amid unsure regulatory expectations, many main organizations are setting the usual by integrating inclusive practices into their trial operations. To drive significant and measurable influence, these efforts should turn into commonplace, persistently utilized, and strengthened by clear accountability throughout the analysis ecosystem.
Rebecca Johnson, PhD, is a medical analysis recruitment and inclusion government and strategist. In her most up-to-date position, Johnson led the medical trial recruitment and variety, fairness, and inclusion features at SPARC. Previous to that, she held a number of management positions inside IQVIA’s affected person recruitment group, together with advising pharmaceutical sponsor groups with their efforts to realize extra numerous trial populations. Johnson has a Grasp’s in Public Well being and a PhD in Well being Sciences. Her doctoral dissertation analysis targeted on growing entry to medical trials for traditionally underrepresented populations.
References
1. Kulkarni, D. (2023, December). The evolution of US FDA variety necessities in medical analysis. DIA World Discussion board. https://globalforum.diaglobal.org/challenge/december-2023/the-evolution-of-us-fda-diversity-requirements-in-clinical-research-2/
2. Coakley, M., Fadiran, E., Parrish, L., Griffith, R., Weiss, E., & Carter, C. (2012). Dialogues on diversifying medical trials: Profitable methods for participating ladies and minorities in medical trials. Journal of Ladies’s Well being, 21(7), 713-716.
3. The Editors. (2021, September 13). Striving for variety in analysis research. The New England Journal of Drugs, 385(15),1429-1430. 10.1056/NEJMe2114651
4. United States Division of Well being and Human Providers (USHHS), Meals and Drug Administration (2025, March 21). FDA-led affected person targeted drug improvement (PFDD) public conferences. https://www.fda.gov/{industry}/prescription-drug-user-fee-amendments/fda-led-patient-focused-drug-development-pfdd-public-meetings
5. United States Division of Well being and Human Providers (USHHS), Meals and Drug Administration (2025, March 21). FDA patient-focused drug improvement steering sequence for enhancing the incorporation of the affected person’s voice in medical product improvement and regulatory resolution making. https://www.fda.gov/medication/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical
6. Meals and Drug Administration Security and Innovation Act, Public Legislation No 112-144, S. 3187, 21 U.S.C. 301 et seq. (2012).
7. Reaney, M. (2025). Affected person centricity within the biopharmaceutical {industry}: Are we almost there but? A set of views from researchers, approvers, and sufferers. DIA World Discussion board. https://globalforum.diaglobal.org/challenge/december-2023/the-evolution-of-us-fda-diversity-requirements-in-clinical-research-2/
8. Bristol Myers Squibb. (2024, August 1). Variety in medical trials is a scientific crucial. https://www.bms.com/media/media-library/scientific-media-resources/importance-of-clinical-trials-and-role-of-diversity.html
9. Grey, S. (2022, January 12). Medicaid protection of “routine prices” for medical trials. Utilized Medical Trials. https://www.appliedclinicaltrialsonline.com/view/medicaid-coverage-of-routine-costs-for-clinical-trials
10. GovTrack.US. (2025, Could 19). S. 4742 (116th): Medical therapy act. GovTrack.US. https://www.govtrack.us/congress/payments/116/s4742/textual content
11. Equitable Breakthroughs in Drugs Growth (EQBMED). (2024, September 12). Equitable breakthroughs in drugs improvement (EQBMED) to work with inaugural sponsor corporations to drive higher variety in medical trials. PR Newswire.https://www.prnewswire.com/news-releases/equitable-breakthroughs-in-medicine-development-eqbmed-to-work-with-inaugural-sponsor-companies-to-drive-greater-diversity-in-clinical-trials-302246462.html
12. Hartnett, Ok. (2023, November 13). Anti-‘woke’ backlash forces well being {industry} to adapt DEI efforts. Trendy Healthcare. https://www.modernhealthcare.com/hospital-systems/healthcare-dei-anti-woke-law-ron-desantis-trinity-health
13. Desilva, H. (2025, January 30). CommonSpirit to broaden DEI packages regardless of federal pushback. Trendy Healthcare. https://www.modernhealthcare.com/suppliers/commonspirit-dei-program-extensions-morehouse
14. The MRCT Heart of Brigham and Ladies’s Hospital and Harvard. (2024). Illustration in analysis. The MRCT Heart of Brigham and Ladies’s Hospital and Harvard.https://dev.mrctcenter.org/mission/representation-in-research/
15. Meals and Drug Administration. (2024, June). Variety Motion Plans to enhance enrollment of members from underrepresented populations in medical research: Steering for {industry}. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-action-plans-improve-enrollment-participants-underrepresented-populations-clinical-studies
16. Nationwide Academies of Sciences, Engineering, and Drugs. (2024). Towards a framework to enhance variety and inclusion in medical trials: Proceedings of a workshop (2024). Washington, DC: The Nationwide Academies Press. https://doi.org/10.17226/28587
17. Nationwide Academies of Sciences, Engineering, and Drugs. (2022). Enhancing illustration in medical trials and analysis: Constructing analysis fairness for ladies and underrepresented teams. Washington, DC: The Nationwide Academies Press. https://doi.org/10.17226/26479
18. Getz, Ok., Smith, Z., & Peña, Y. (2020). Quantifying affected person subpopulation disparities in new medication and biologics authorised between 2007 and 2017. Therapeutic Innovation & Regulatory Science, 54(6), 1541-1550. 10.1007/s43441-020-00181-9
19. Smith, Z., Botto, E., Johnson, O., Rudo, T., & Getz, Ok. (2024). New benchmarks on demographic disparities in pivotal trials supporting FDA-approved medication and biologics. Therapeutic Innovation & Regulatory Science, 58(1), 143-152. 10.1007/s43441-023-00579-1
20. Peroutka, S. (2024). Demographic comparability of topics in FDA-approval trials in america to dysfunction particular demographics utilizing Actual World Information. Modern Medical Trials Communications, 39:e101293. 10.1016/j.conctc.2024.101293
21. Reid, M., Davis, S., Henry, O., Mathew, A., McCallister, S., Nero, T., Rabheru, S., Sampson, S., Vanderslice, T., & Williams, D. (2023). Demographic variety of US-based members in GSK-sponsored interventional medical trials. Medical Trials, 20(2), 133-144
22. Exec. Order No. 14173, 3 C.F.R. 8633-8636 (2025). https://www.govinfo.gov/content material/pkg/FR-2025-01-31/pdf/2025-02097.pdf
23. Exec. Order No. 14151, 3 C.F.R. 8339-8341 (2025). https://www.govinfo.gov/content material/pkg/FR-2025-01-29/pdf/2025-01953.pdf
24. Exec. Order No. 14168, 3 C.F.R. 8615-8618 (2025). https://www.govinfo.gov/content material/pkg/FR-2025-01-30/pdf/2025-02090.pdf
25. Seminera, M. (2025, March 8). Universities are dealing with large cuts to analysis funding. At Duke, it’s a time for ‘harm management’. Related Press. https://apnews.com/article/trump-cuts-research-funding-nih-duke-7f24b33bbad54490583520536ab40e0c
26. Constantino, A. (2025, April 30). RFK Jr. is gutting minority well being places of work throughout HHS which are key to lowering well being disparities. CNBC. https://www.cnbc.com/2025/04/30/rfk-jr-hhs-job-cuts-minority-health-offices.html
27. Whitlock, J. (2025, Could 1). US drugmakers more and more look overseas for medical trials as confidence in FDA wavers. EndpointsNews. https://endpts.com/us-drugmakers-seek-clinical-trials-abroad-as-confidence-in-fda-wavers/
28. Fick, M. (2025, Could 14). Upheaval at FDA pushes some biotech corporations to maneuver early trials out of US. Reuters. https://www.reuters.com/sustainability/boards-policy-regulation/fda-upheaval-pushes-some-biotech-firms-plan-early-trials-out-us-2025-05-14/
29. PhRMA. (n.d.) The inflation discount act and Medicare drug worth “negotiation.” https://phrma.org/policy-issues/government-price-setting/inflation-reduction-act
30. United States Division of Well being and Human Providers (USHHS), Nationwide Institutes of Well being. (2024, October 21). NIH insurance policies and pointers on the inclusion of ladies and minorities as topics in medical analysis. https://grants.nih.gov/policy-and-compliance/policy-topics/inclusion/women-and-minorities/guideline
31. Durant, R., Davis, R., George, D., Williams, I., Blumenthal, C., & Corbie-Smith, G. (2007). Participation in analysis research: Components related to failing to satisfy minority recruitment objectives. Annals of Epidemiology, 17(8), 634-642.
32. Boden-Albala, B., Carman, H., Southwick, L., Parikh, N., Roberts, E., Waddy, S., & Edwards, D. (2015). Inspecting boundaries and practices to recruitment and retention in stroke medical trials. Stroke, 46(8), 2232-2237.
33. Choradia, N., Karzai, F., Nipp, R., Naqash, A., Gulley, J., & Floudas, C. (2024). Growing variety in medical trials: Demographic tendencies on the Nationwide Most cancers Institute, 2005-2020. Journal of the Nationwide Most cancers Institute, 116(7), 1063-1071.
34. United States Division of Well being and Human Providers (USHHS), Nationwide Institutes of Well being, Workplace of Extramural Analysis. (2018, January). Alzheimer’s illness analysis facilities (Funding alternative announcementRFA-AG-19-001).https://grants.nih.gov/grants/information/rfa-files/RFA-AG-19-001.html

